Abstract
Bosutinib is a tyrosine kinase inhibitor (TKI), approved for adults with Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML); 400 mg in newly diagnosed (ND) patients (pts), and 500 mg in resistant/intolerant (R/I) pts, administered once daily (QD) with food. Its toxicity profile differs from other TKIs approved in children (imatinib, dasatinib and nilotinib), showing a higher incidence of gastrointestinal (GI) adverse events (AEs) but fewer toxicities such as musculoskeletal AEs (Cortes JE et al, 2016). Furthermore, in mice, bosutinib showed less growth impairment compared to other TKIs (Tauer JT et al, 2015).
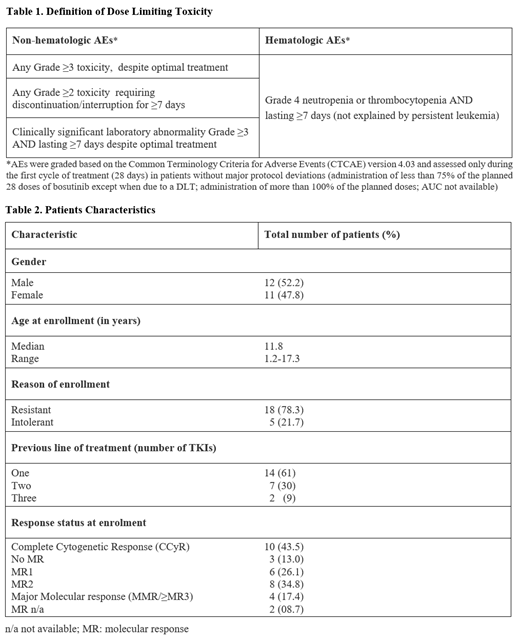
Study NCT04258943 is an international, open-label, phase I/II trial, initially enrolling pediatric Ph+ CML R/I pts (per 2013 European Leukemia Net criteria), in chronic, accelerated or blastic (AP/BP) phase, aged ≥1 and <18 years, with normal liver and kidney function. Main exclusion criteria consisted of leptomeningeal leukemia in AP/BP pts, T315I or V299L BCR-ABL1 mutations, use of proton pump inhibitors and moderate/strong CYP3A inducers/inhibitors. The protocol was amended to enroll ND pts in the phase II. Results from the phase I in R/I pts, with a data cut-off 05 July 2021, are presented. The primary objective was to determine the recommended phase II dose (RP2D) of bosutinib in R/I and ND pts. Secondary objectives included safety and preliminary efficacy. A 6+4 dose-escalation design was used. The RP2D was defined as the dose resulting in 0/6 or 1/10 dose limiting toxicities (DLTs; see table 1) in pts who received ≥ 75% of the planned dose in cycle 1, and mean area under the curve (AUC) of 3150 (±20%) ng·hr/mL in R/I pts and 2270 (±20%) ng·hr/mL in ND pts (equivalent to the exposure of 500 mg and 400 mg QD in adults, respectively). Cycles consisted of 28 days of bosutinib QD, starting from 300 mg/m 2, with escalation to 350 mg/m 2 and 400 mg/m 2 (max 600 mg QD). Pharmacokinetic sampling was performed on day 14 of cycle 1, after achievement of steady-state. All protocol versions obtained ethics approval. The study is sponsored by Erasmus Medical Center in Europe and COG in the United States, it is open in 19 ITCC and 28 COG sites and is funded by Pfizer.
Since November 15 2016, 23 pts with R/I CP CML were enrolled at 12 ITCC and 2 COG centers. Pts characteristics are provided in table 2. Six pts were enrolled at 300 mg/m 2 (median n. of cycles 18.5; range 11-57), without DLTs. The dose was escalated to 350mg/m 2 and 11 pts were enrolled (median n. of cycles 10, range 1-25); 1 DLT occurred (grade 3 GI toxicity). At 300 mg/m 2 (AUC: 2010 ng·hr/mL) and 350 mg/m 2 (AUC: 2385 ng·hr/mL) the AUC was under target for R/I pts; but the 300 mg/m 2 dose resulted in adequate and safe exposure for ND pts. For R/I pts the dose was increased to 400mg/m 2 and 6 pts were enrolled (median n. of cycles 3, range 0-7). One patient experienced a DLT (Grade 3 transaminase increase, rash and treatment interruption > 7 days). Exposure at 400mg/m 2 (n=4) was within the target interval at 3064 ng·hr/mL. Overall, 22/23 pts had at least 1 AE, most common were GI toxicities (87%) and rash (39%). Grade 3 or 4 AEs were observed in 10/23 (43.5%) pts; GI were the most common (6/10 pts). Elevated transaminases occurred in 6 pts, 3 of which at grade 3 or 4. Median follow up was 9.9 months (mns). Including pts in Complete Cytogenetic Response/Major Molecular response (CCyR/MMR) at screening, cumulative incidence of CCyR at 3 and 6 mns was 68% (95% CI: 49-88%) and 82% (95% CI: 66-98%); the cumulative incidence of MMR at 3 and 6 mns was 27% (95% CI: 09-46%) and 36% (95% CI: 16-56%) respectively. Considering only pts that achieved CCyR/MMR during treatment, at 3 and 6 mns, 4/13 and 3/9 pts achieved CCyR (median time to response 3 mns; range 2.5-5.7) while 3/19 and 2/17 achieved MMR (median time to response 3 mns; range 2.5-16.5), respectively. All patients that achieved or entered the study in CCyR and/or MMR (table 2) maintained the response. Four pts stopped the treatment due to insufficient response.
In ND pts, the RP2D was established at 300 mg/m 2 QD. In R/I pts, 400 mg/m 2 QD seems to result in comparable exposure as 500 mg QD in adults, however the cohort is to be completed to establish the RP2D. The AE profile of bosutinib was consistent with the known safety profile in adults. GI toxicities were the most common, mainly during the first cycles and tended to improve in subsequent cycles. Cumulative MMR and CCyR rates were comparable to those reported in literature for other TKIs (Hijiya N et al, 2019).
Durairaj: Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Viqueira: Pfizer: Current Employment, Current equity holder in publicly-traded company. Ingrosso: Pfizer: Current Employment, Current equity holder in publicly-traded company. Locatelli: Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Miltenyi: Speakers Bureau; Medac: Speakers Bureau; Jazz Pharamceutical: Speakers Bureau; Takeda: Speakers Bureau. Hijiya: Novartis: Consultancy; Stemline Therapeutics: Consultancy. Zwaan: SANOFI: Consultancy; NOVARTIS: Consultancy; ROCHE: Consultancy; INCYTE: Consultancy; PFIZER: Consultancy, Research Funding; JAZZ: Other: travel funding, Research Funding; BMS: Research Funding; Abbvie: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal